How Do I Report?
Consumer reporting is how we work together, as one people, to improve everything from cars to toys to furniture to vaccine medicine. Silence helps no one.
“It’s just my imagination.”
“I’m probably wrong.”
“Correlation doesn’t prove causality.”
Even if…all three of those above statements are true (and the 3rd one absolutely is), reporting an adverse reaction following vaccination is the absolute best choice you can make. For yourself, your family and your community.
Purely from the perspective of consumer protections…there is only harm that can come from the under-reporting of drug reactions. Especially when the under-reporting is severe (90%+) and chronic, as is the case with consumer feedback regarding vaccine medicine (32+ years).
On February 10th, we launched Reporting Hesitancy Project Survey #1. If you’ve just come from there, thank you & welcome!
What survey, you ask? This One!
Please take a few minutes to participate in our survey, helping us better understand the inhibitions most confront when experiencing an adverse reaction following vaccination. Even me! My own son’s adverse reaction (and lifelong anaphylactic injury) also went unreported. Why?
If we solve that question, we will reverse the severe problem of “under-reporting.” This is how we will gain improved and better medical knowledge around vaccines.
Feel overwhelmed already? That may be intentional, BUT…the “rumors” of how challenging and difficult these systems may be to work with are likely both true and sometimes inflated. What feels impossible for a single mother of an injured child with no support is perhaps not challenging at all for a mother who is happily married, works as a lawyer and has the backing of her child’s pediatrician.
This is why I’ve also written a stack article called Community Resources that helps people connect with community organizations and citizen advocacy groups. If you need help, support, to simply be seen and believed, maybe a shoulder to cry on, or the opportunity to hear other people’s stories and realize you are not alone…these groups have your back.
And now, by country…
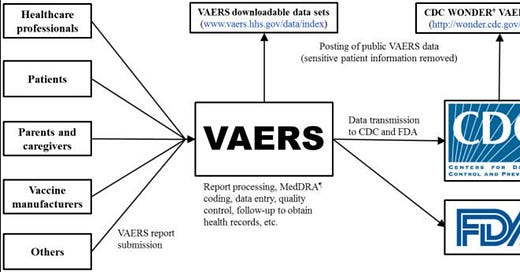
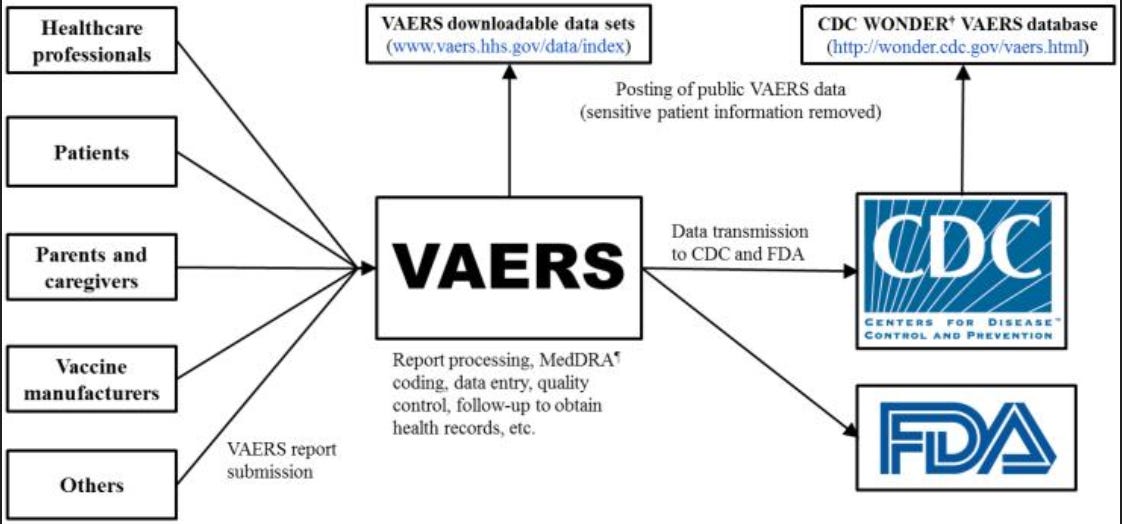
USA - Vaccine Adverse Events Reporting System (VAERS)
https://vaers.hhs.gov/ — (or click VAERS above)
The Vaccine Adverse Event Reporting System (VAERS) is a passive reporting system, meaning it relies on individuals to send in reports of their experiences. Anyone can submit a report to VAERS, including parents and patients.
Please Note: Healthcare providers are required by law to report to VAERS certain adverse events, and healthcare providers are strongly encouraged to report to VAERS any adverse event that occurs after the administration of a vaccine licensed in the United States, whether it is or is not clear that a vaccine caused the adverse event.
Canada - Canadian Adverse Events Following Immunization Surveillance System (CAEFISS)
https://www.canada.ca/en/public-health/services/immunization/canadian-adverse-events-following-immunization-surveillance-system-caefiss.html — (or click CAEFISS above)
The Canadian Adverse Events Following Immunization Surveillance System (CAEFISS) is a federal, provincial and territorial (FPT) public health post-market vaccine safety surveillance system. CAEFISS is managed by PHAC and is unique in that it includes both passive (spontaneous reports from FPTs) and active surveillance.
CAEFISS reports are submitted by public health authorities in provinces and territories, who in turn receive them from local public health units. Provincial and territorial authorities also receive reports from federal authorities that provide immunization within their jurisdiction, including: the RCMP, Indigenous Services Canada, and Correctional Service Canada.
United Kingdom - Medicines & Healthcare products Regulatory Agency (Yellow Card)
https://yellowcard.mhra.gov.uk/ — (or click Yellow Card above)
Welcome to the new Yellow Card reporting site
Report suspected side effects to medicines, vaccines, e-cigarettes, medical device incidents, defective or falsified (fake) products to the Medicines and Healthcare products Regulatory Agency to ensure safe and effective use.
The MHRA works with five regional Yellow Card Centres to increase awareness as well as educate and promote the reporting of suspected side effects to the Yellow Card scheme with healthcare professionals, patients and their representative organisations. Yellow Card Centres can help you or your organisation improve awareness of the scheme and to help encourage others to report. Contact Us Here.
European Union - European Medicines Agency (EMA)
https://www.ema.europa.eu/en/human-regulatory/research-development/pharmacovigilance/eudravigilance — (or click EMA above)
EudraVigilance is the system for managing and analysing information on suspected adverse reactions to medicines which have been authorised or being studied in clinical trials in the European Economic Area (EEA). The European Medicines Agency (EMA) operates the system on behalf of the European Union (EU) medicines regulatory network.
Please Note: From 30 June 2022, it will be mandatory to report side effects to EudraVigilance using a data format based on international standards set by the International Organization for Standardization (ISO). This will help increase the data quality and analytical capabilities in EudraVigilance.
Australia - Therapeutic Goods Administration (TGA)
https://www.tga.gov.au/reporting-adverse-events (or click TGA above)
Adverse events are unintended and sometimes harmful occurrences associated with the use of a medicine, vaccine or medical device (collectively known as therapeutic goods). Adverse events include side effects to medicines and vaccines, and problems or incidents involving medical devices.
The TGA monitors adverse events (such as side effects) related to medicines and vaccines to safeguard and enhance the health of the Australian community. Unfortunately it's impossible to know all potential adverse events of a medicine or vaccine before it is approved for use. When people tell us about their experiences using a particular medicine or vaccine, it helps us to monitor the safety of those products. Report Online & Get Advice Here.
New Zealand — Medical Device Adverse Event Reporting (MEDSAFE)
https://www.medsafe.govt.nz/regulatory/DevicesNew/9AdverseEvent.asp (or click MEDSAFE above)
Anyone can report an issue associated with a medical device. An issue may relate to an adverse event, or a quality issue. Patients, caregivers, healthcare professionals and suppliers are all encouraged to lodge an adverse event report.
An adverse event is an unintended consequence associated with the use of a medical device or with an implanted medical device. By reporting these to Medsafe, seemingly isolated incidents may be collated and responded to. You do not have to be certain that the medical device caused the adverse event, just suspicious. When we receive a number of reports with similar events it helps us to determine if the medical device plays a role in the adverse event.
I’ll be honest, the New Zealand system was the hardest to understand. If you encounter trouble, I recommend searching for members of your community organized into support groups.
Forgive us, but we’ve started with the communities we know best (Western World w/ English Fluency). Eventually, I would love (love, love, love!) to collaborate with groups throughout South America, Africa, the Middle East, Eastern Europe, Russia and Asia…but that’s for the future.
Please share this article widely, as I’ve written it to appeal to everyone. Each of us, in seeking the benefits of vaccine medicine, knowingly (or unknowingly) accept the potential risks. When an injury occurs, it’s not our fault. It is a problem, and it is both our right and our duty to file a report. By doing so, we help shine a light on possible trends, bad batches and other signals of concern. This guides investment dollars (and our best scientists) to the drawing table, where they can reconsider and improve vaccine medicine. Science evolves, if it is science at all.
And remember. Before Covid and government threats, pressure, mandates and coercion…every vaccine injured person, chose to get vaccinated. Historically, the injured were either children with no choice, or adults who believed/trusted in vaccines.
The tragedy of the vaccine injured being scorned by society, shunned and silenced, is one almost too painful to bear. Please keep this in mind, when a friend or family member confides in you.
The Reporting Hesitancy Project is our chance to push the pedal to the metal on the issue of under-reporting. I’m bringing 25 years of experience to this project and I’d like to die knowing I helped bring about a sea change to this tragedy of under-reporting. We must close the patient/consumer feedback loop, so investment dollars can be directed toward solving the very real problems that exist.
Please help me do this by hitting the SHARE arrow. ❤️ Many thanks!